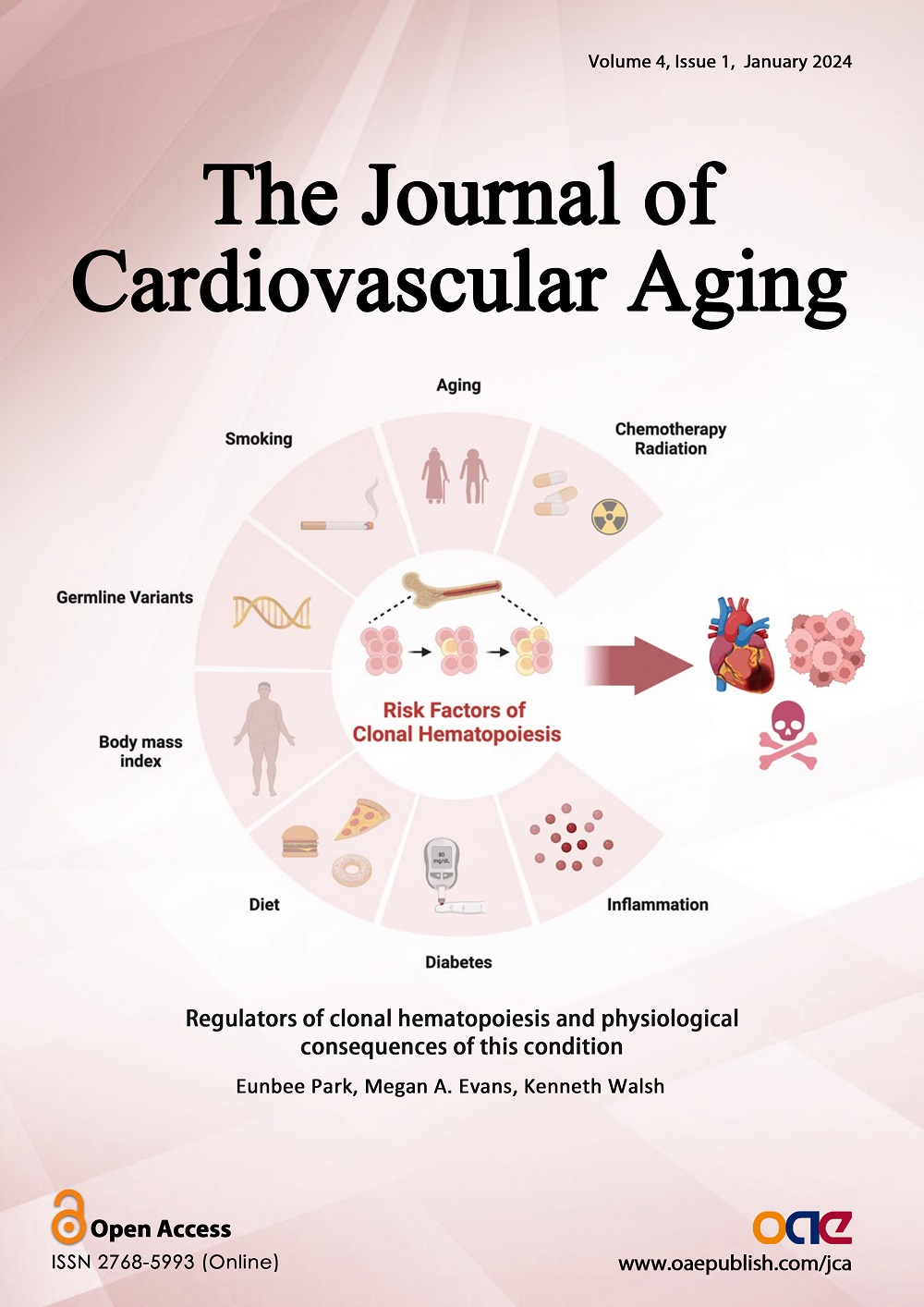
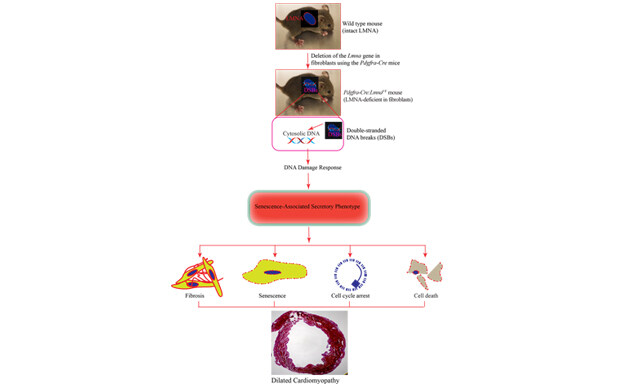
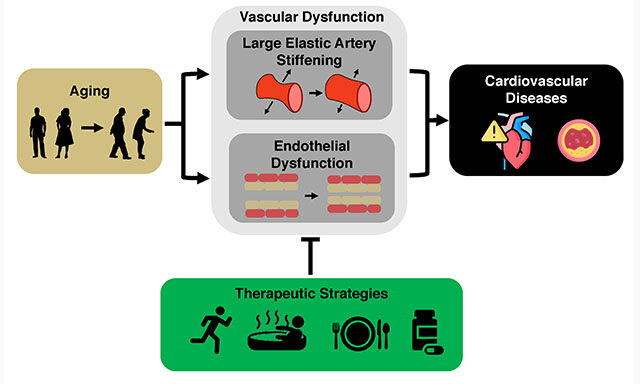
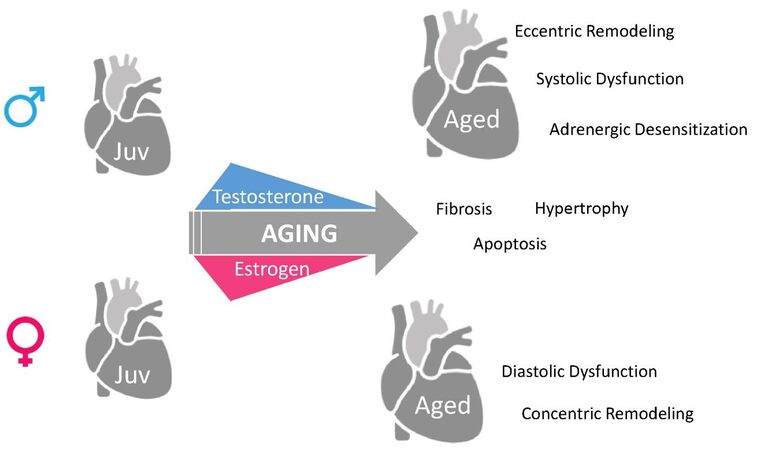
The Journal of Cardiovascular Aging

Topic: Stem Cell & Genomics From Precision Medicine to Clinical Trial in Dish
NaN
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Views: Downloads:
Data
520
Authors
86
Reviewers
2021
Published Since
132
Citations
178,394
Article Views
75,195
Article Downloads
For Reviewers
For Readers
Add your e-mail address to receive forthcoming Issues of this journal:
Themed Collections

Topic: Stem Cell & Genomics From Precision Medicine to Clinical Trial in Dish
NaN
Data
520
Authors
86
Reviewers
2021
Published Since
132
Citations
178,394
Article Views
75,195
Article Downloads