Male carriers of HLA-C*04:01 have increased risk of cardiac injury in COVID-19
Abstract
Identification of factors that lead to the severe clinical course of COVID-19 is crucial for timely allocation of resources. The purpose of this study was to evaluate possible sex differences in cardiac injury associated with HLA-C*04:01. High sensitivity troponin T on admission (hs-TnTa) and maximum high sensitivity troponin T (hs-TnTmax) were used to assess for cardiac injury in patients with COVID-19 (n = 435). We tested for the association of elevated hs-TnT with HLA-C* 04:01 and evaluated for potential sex-specific differences. An association between hs-TnTa and the severity of clinical course was identified. In addition, our study revealed that hs-TnTmax was higher in men who were carriers of HLA-C*04:01 compared to men without the risk allele. Male carriers of HLA-C*04:01 with COVID-19 developed higher hs-TnTmax, suggesting a larger extent of cardiac injury. This association suggests the presence of different pathomechanisms in COVID-19 based on sex.
Keywords
INTRODUCTION
Since November 2019, the rapid spread of coronavirus disease 2019 (COVID-19) has become one of the greatest challenges to the healthcare system in the 21st century.
Identification of genetic or acquired risk factors is crucial to prevent or mitigate adverse outcomes.
We recently reported on HLA-C*04:01, a potential risk gene for the severe clinical course of COVID-19. HLA-C*04:01 carriers had double the risk of intubation [odds ratio 3.5 (CI 95% 1.9-6.6; risk ratio 1.5
There is a broad spectrum of clinical manifestations of COVID-19. In 7.2%-12% of cases, the myocardial injury was detected[2,3] and reported to be associated with a worse prognosis[2-5]. Changes in high sensitivity troponin T (hs-TnT) levels over time may suggest cardiac injury at any point during hospitalization[6-9]. Therefore, serial hs-TnT testing might be useful in staging disease severity[7]. Since male sex was reported as an independent risk factor for a severe clinical course of COVID-19[10], we performed a subgroup analysis of our cohort based on sex.
METHODS
In the spring of 2020, an international multicenter investigation was initiated to analyze the association of human leukocyte antigens (HLA) with the severe course of COVID-19. In total, 435 patients from Germany (n = 135), Switzerland (n = 20), Spain (n = 133), and the United States (n = 147) were included in the study and HLA typed.
Enrolled patients were older than 18 years with COVID-19 confirmed by real-time quantitative polymerase chain reaction method and represented the complete clinical spectrum from mild to severe cases. The severe clinical course of COVID-19 was defined as requiring intubation. Patients were recruited from the outpatient clinics, emergency rooms, inpatient wards, and intensive care units. Results of HLA analyses were replicated in genome-wide association studies (GWAS). HLA-C*04: 01 was identified as a risk factor for a severe course of COVID-19 and for susceptibility to COVID-19, as previously described[1].
Then, the relationship between hs-TnT on admission (hs-TnTa) or the maximum hs-TnT (hs-TnTmax) value during the clinical treatment period and HLA-C*04:01 carrier status was investigated. Hs-TnT values > 0.14 ng/L were considered pathologic according to hospital laboratory cut-offs.
Patients for whom hs-TnT values were available were included in the subgroup analysis (n = 104). After examining the normal distribution, the cohorts were compared using the Wilcoxon-Mann-Whitney-Test. A P-value of < 0.05 was considered statistically significant. Correction for multiple testing was carried out using the Bonferroni method. In addition, creatine phosphokinase (CK) levels were analyzed for all patients within the hs-TnT subgroup analysis. The same methodology as for hs-TnT values was used.
This study was approved by the ethics committee of Charité-Universitätsmedizin-Berlin (EA2/066/20). Informed consent was obtained from all patients prior to inclusion in the study.
RESULTS
The cohort analyzed consisted of 104 patients, including 27 females (26%). The median age was 62.5 years (IQR 14 years) with a median BMI of 28 kg/m² (IQR 7 kg/m²). Pre-existing cardiovascular diseases and risk factors included arterial hypertension (58%), diabetes mellitus (all types, 29%), and coronary artery disease (13%).
An association between hs-TnTa and the severity of clinical course of COVID-19 (P < 0.001) was identified. This association was independent of the HLA-C*04:01 carrier status of the patient. There was no difference in the concentration of hs-TnTa in men vs. women, and an age-stratified analysis showed no significant differences.
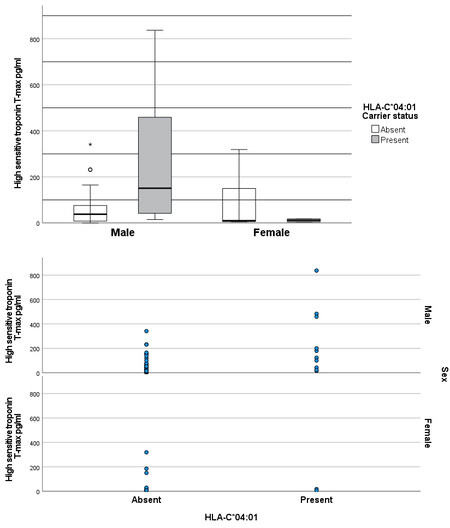
However, hs-TnTmax was higher in male carriers of HLA-C*04:01 (n = 14, median hs-TnTmax 151 pg/mL) vs. men without the risk allele (n = 63, median hs-TnTmax 38 pg/mL; P = 0.008; Figure 1). The result remained significant after excluding the patient with the highest hs-TnTmax value of 837 pg/mL (P = 0.013 vs.P = 0.008 in the analysis of the complete data set; Figure 1, dot plot).
Figure 1. hs-TnTmax based on HLA-C*04:01 carrier status and sex. ○ and * symbolize statistical outliers. A ○ marks statistical outliers that are within 1.5-3.0× of the interquartile range (IQR). A * marks statistical outliers that are > 3.0× the IQR.
There was no difference in cardiovascular risk factors, such as diabetes mellitus, arterial hypertension, and coronary artery disease, between male carriers and non-carriers of HLA-C*04:01 [Table 1], suggesting that the allele was an independent risk factor. In women, there was no difference in hs-TnTmax between carriers and non-carriers of HLA-C*04:01. An age-stratified analysis showed no significant differences.
Baseline parameters and hs-TnTa and hs-TnTmax based on sex and HLA-C*04:01 status. *P < 0.05
| HLA-C*04:01 | ||||
| Carrier | Non carrier | Non carrier | Carrier | |
| Male n = 14 | Male n = 63 | Female n = 22 | Female n = 5 | |
| Age in years; median (IQR) | 63 (21) | 62 (16) | 64 (16) | 54 (9) |
| BMI in kg/m2; median (IQR) | 26 (4) | 28 (6) | 31 (13) | 27 (3) |
| Medical History | ||||
| CAD n (%) | 1 (7%) | 12 (19%) | 1 (5%) | 0 (0%) |
| Diabetes mellitus; n (%) | 5 (36%) | 20 (32%) | 4 (18%) | 2 (40%) |
| Art. Hypertension; n (%) | 9 (64%) | 34 (54%) | 14 (64%) | 3 (60%) |
| Acute kidney failure n (%) | 2 (14%) | 4 (6%) | 0 (0%) | 0 (0%) |
| Clinical Events | ||||
| Intensive care unit n (%) | 10 (71%) | 38 (60%) | 7 (32%) | 2 (40%) |
| Mechanical ventilation n (%) | 10 (71%) | 22 (35%) | 5 (23%) | 1 (20%) |
| Died n (%) | 2 (14%) | 7 (11%) | 1 (5%) | 0 (0%) |
| Laboratory Data | ||||
| Erythrocytes/pl median (IQR) | 4,2 (1,0) | 4,5 (1,2) | 4,5 (0,9) | 4,4 (1,2) |
| Hemoglobin (Hb) g/l median (IQR) | 12,6 (2,7) | 13,3 (3,8) | 12,5 (2,8) | 13,5 (3,9) |
| Hematocrit (Hct) % median (IQR) | 37 (6) | 38 (10) | 36 (8) | 37 (10) |
| Leukocytes 1000/nl median (IQR) | 10,2 (7,7) | 7,0 (4,1) | 5,9 (3,8) | 8,1 (2,2) |
| Lymphocytes /nl median (IQR) | 1,6 (2,0) | 0,9 (0,5) | 0,9 (0,7) | 1,1 (0,1) |
| Thrombocytes 1000/nl median (IQR) | 170 (83) | 194 (119) | 215 (68) | 242 (26) |
| Procalcitonin (PCT) µg/l median (IQR) | 0,5 (1,3) | 0,2 (0,4) | 0,1 (0,1) | 0,2 (0,2) |
| C-reactive protein (CRP) mg/L median (IQR) | 142 (205) | 79 (100) | 50,5 (115) | 92 (5) |
| Myoglobin µg/l median (IQR) | 264 (384) | 133 (561) | 921 (0) | 21 (0) |
| Creatine phosphokinase (CK) U/l median (IQR) | 273 (801) | 117 (238) | 82 (51) | 39 (52) |
| hs-TnTa pg/mL median (IQR) | 22,0 (68,0) | 18,0 (38,5) | 8,5 (17,0) | 13,0 (11,0) |
| hs-TnTmax pg/mL median (IQR) | 151,0 (417,0)* | 36,0 (68,0) | 10,0 (144,0) | 11,5 (13,0) |
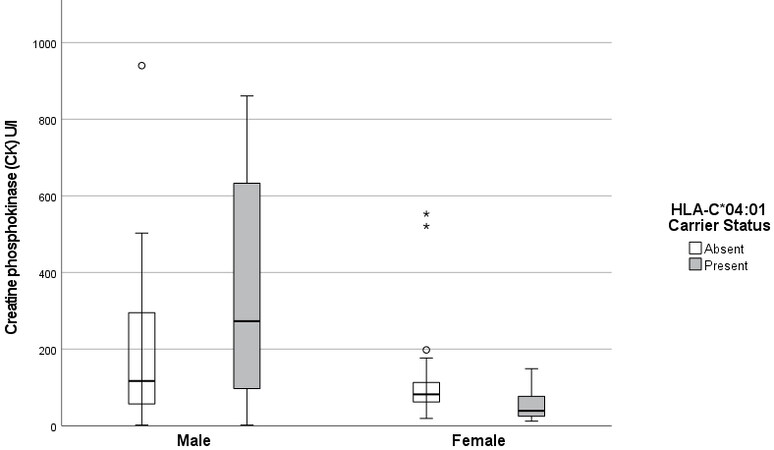
After that, CK was analyzed as an additional marker for cardiac injury. Figure 2 illustrates a trend of higher CK blood levels in male carriers of HLA-C*04:01 vs. male non-carriers. This trend did not reach statistical significance. However, male carriers of HLA-C*04:01 had higher CK levels than female carriers of
DISCUSSION
Male sex has been shown to be an important clinical risk factor for adverse outcomes in COVID-19[11]. We recently described HLA-C*04:01 as a new risk factor for the severe clinical course of COVID-19[1]. The main hypothesis of our current study is that carriers of HLA-C*04:01 have an increased risk for cardiac injury and that there are sex-specific differences with regards to the extent. We performed a subgroup analysis of
The general pathomechanisms of SARS-CoV-2 are well described in the literature. To enter a cell, SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor, which has been described in various tissues including adipose tissue, intestinal tract, kidney, lungs, central nervous system, and the cardiovascular system, particularly in the endothelium[12]. As a result, endothelial injury and associated myocardial injury are commonly observed in patients with COVID-19[13]. In that regard, hormones play a crucial role in the regulation of the renin-angiotensin-aldosterone system (RAAS) and, consequently, ACE2 and its membrane-bound form, the ACE2 receptor. While the effects on COVID-19 are not yet fully understood, testosterone is known to increase ACE2 in the heart and kidneys, while estrogen decreases ACE2 in the same organs. Data on the course and severity of COVID-19 also support the notion of potential biological sex differences affecting COVID-19 vulnerability, as 50% more men than women were hospitalized during outbreaks[14]. Moreover, the male-to-female case fatality ratio seems to increase in all age groups[14].
The results of this study are consistent with existing evidence. The association of HLA-C*04:01 with hs-TnT values in male carriers is described herein for the first time.
Similar to prior studies, an association between hs-TnTa and the severity of clinical course of COVID-19 was identified[11,15].
Guadiana-Romualdo et al. identified hs-TnT as an independent predictor for 30-day mortality in
In their multicenter, cross-sectional study, Lombardi et al. also found that hs-TnT was an independent variable associated with increased in-hospital mortality independent of concomitant cardiac diseases during COVID-19[15]. Furthermore, the risk for cardiovascular and non-cardiovascular complications such as heart failure, multiorgan failure, pulmonary embolism, and sepsis was increased in the group with elevated
A possible explanation for sex-specific differences in hs-TnT elevation may be hormonal factors. Estradiol has been demonstrated to enhance T-cell response against infectious agents in general and increase antibody production, somatic hypermutation, and class switching[16]. Estradiol promotes increased levels of neutrophils and stimulates the release of cytokines by monocytes/macrophages[17]. Furthermore, the decrease in estradiol levels in postmenopausal women has been associated with increased production of inflammatory cytokines in COVID-19[18].
Estradiol levels decrease post menopause. However, the cardiovascular protective effects of decades of higher estradiol levels in the blood appear to remain for some time. In that regard, Dietl et al. showed the convergence of hs-TnT in men and women post menopause with increasing age[19]. Subramanya et al. demonstrated an increase in the left ventricular mass (LVM) as a result of an increasing androgen effect in postmenopausal women[20]. These findings provide a biologically plausible explanation for the increase in cardiac markers in men with COVID-19.
Given the small sample size of our cohort, an analysis of pre- and postmenopausal women was not feasible. Further research is needed to study the influence of HLA-C*04:01 in women with COVID-19 before and after menopause.
The increase of hs-TnTmax in male carriers of HLA-C*04:01 was accompanied by a trend of higher CK values [Figure 2], supporting the hypothesis that these individuals are at greater risk for cardiac injury compared to male non-carriers.
Renal function may impact serum levels of hs-TnT and should be considered in the analysis[21]. In our cohort of male carriers of HLA-C*04:01, 14% (n = 2) had acute kidney failure. When those two patients were excluded from the analysis, the results remained significant.
In conclusion, the correlation of hs-TnTmax elevation in combination with HLA-C*04:01 carrier status in men supports our hypothesis of a sex-specific increase in the risk for cardiac injury. The results suggest a potential sex difference in the pathomechanism of COVID-19. Our findings may help to better understand the pathophysiology of COVID-19 and define more targeted therapy concepts for risk groups. Due to the relatively small number of patients, it is necessary to validate the results in a larger cohort.
Limitations
Sample size and inclusion of different stages of COVID-19 at the time of admission should be considered as limitations of the study. The patients included were mainly of European ethnicity. Larger and multiethnic cohorts are necessary to reliably investigate the exact size of the effects. Furthermore, a comparison of pre- and postmenopausal women will be necessary to investigate potential hormonal influences in more detail.
DECLARATIONS
Authors’ contributionsContributed to the conception of the study: Suwalski P, Poller W, Kurth F, Guettouche T, Landmesser U, Heidecker B
Contributed to the analysis and interpretation of data: Suwalski P, Violano M, Guettouche T, Heidecker B
Conducted the laboratory processing and data preparation: Suwalski P, Violano M, Thibeault C, Quedenau C, Wang X, Karadeniz Z, Tatiana B, Heidecker B
Contributed to study design and data collection: Suwalski P, Violano M, Müller M, Dimitri P, Saccomanno J, Doehn JM, Hübner RH, Hinzmann B, Beer HJ, Wiggli B, Siemann S, Suttorp N, Witzenrath M, Hippenstiel S, Skurk C, Sander LE, Pa-COVID Study Group, Heidecker B
Availability of data and materialsPatient genetic data underlying the study can be made available upon request pending necessary ethics committee and/or confidentiality statements approvals.
Financial support and sponsorshipThis work was supported through research funding from Roche Sequencing Solutions, Inc. and a project grant from the Swiss National Science Foundation issued to Bettina Heidecker, MD (Money Follows Researcher Program). We thank Alicia Vela, Manuela Torres, Gregor Obernosterer, and Jose Munoz from Roche Sequencing Solutions, Inc. for their assistance with project coordination and shipment of samples. This work was supported by the Berlin Institutes of Health (B.I.H. support to the PA-COVID-19 study group, represented by L.E.S and M.W.). Norbert Suttorp, MD is supported by grants from the German Research Foundation, SFB-TR84 C9 and by the German Ministry of Education and Research (BMBF) in the framework of the CAPSyS (01ZX1304B), CAPSyS-COVID (01ZX1604B), SYMPATH (01ZX1906A), PROVID (01KI20160A). Martin Witzenrath, MD is supported by grants from the German Research Foundation, SFB-TR84 C6 and C9, SFB 1449 B2, by the German Ministry of Education and Research (BMBF) in the framework of the CAPSyS (01ZX1304B), CAPSyS-COVID (01ZX1604B), SYMPATH (01ZX1906A), PROVID (01KI20160A) P4C (16GW0141), MAPVAP (16GW0247), NUM-NAPKON (01KX2021), and by the Berlin Institute of Health (CM-COVID). We acknowledge support from the German Research Foundation (DFG).
Conflicts of interestBettina Heidecker, MD reports support from Roche Sequencing Solutions, Inc; a project grant from the Swiss National Science Foundation; is an inventor on patents that use RNA for diagnosis of myocarditis. Juerg H. Beer, MD reports grants from the Swiss National Foundation of Science, the Swiss Heart Foundation, the Foundation Kardio, Baden; Grant support to the institution from Bayer not related to this study; and lecture fee from Daiichi Sankyo to the institution. Martin Witzenrath, MD reports grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kroner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Quark Pharma, Takeda Pharma, Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Actelion, Bayer Health Care, Biotest, and Boehringer Ingelheim; consulting fees from Noxxon, Pantherna, Silence Therapeutics, Vaxxilon, Aptarion, Glaxo Smith Kline, Sinoxa, and Biotest; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Astra Zeneca, Berlin Chemie, Chiesi, Novartis, Teva, Actelion, Boehringer Ingelheim, Glaxo Smith Kline, Biotest, and Bayer Health Care; patent EPO 12,181,535.1: IL-27 for modulation of immune response in acute lung injury issued 2012, patent WO/2010/094,491: Means for inhibiting the expression of Ang-2 issued 2010, and patent DE 102,020,116,249.9: Camostat/ Niclosamide cotreatment in SARS-CoV-2 infected human lung cells issued 2020/21. Melina Muller declares support from Roche Sequencing Solutions and Swiss National Science Foundation and Berlin Institutes of Health. Bernd Hinzmann and Sandra Siemann declare support from Roche Sequencing Solutions. Leif Erik Sander reports Berlin Institutes of Health support to the PA-COVID-19 study group. Wolfgang Poller reports that this study was partially funded by Roche Sequencing Solutions, Inc., which also provided material for exome sequencing. Ulf Landmesser reports consulting fees from Abbott, Amgen, Bayer, Cardiac Dimensions, Novartis, Pfizer, and Omeicos; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Abott, NovoNordisk, Bayer, Amgen, DaiichiSankyo, Pfizer, Sanofi, Boson Scientific, Astra Zeneca, and Boehringer Ingelheim. All other authors have nothing to declare.
Ethical approval and consent to participateThis study was approved by the ethics committee of Charité-Universitätsmedizin-Berlin (EA2/066/20). Informed consent was obtained from all patients prior to inclusion in the study.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Weiner J, Suwalski P, Holtgrewe M, et al. Increased risk of severe clinical course of COVID-19 in carriers of HLA-C*04:01. EClinicalMedicine 2021;40:101099.
2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9.
3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.
4. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-8.
5. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-74.
6. Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367.
7. Lala A, Johnson KW, Russak AJ, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. medRxiv 2020; doi: 10.1101/2020.04.20.20072702.
8. Lorente-Ros A, Monteagudo Ruiz JM, Rincón LM, et al. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J 2020;27:489-96.
9. Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol 2020;76:1244-58.
10. Romiti GF, Cangemi R, Toriello F, et al. Sex-specific cut-offs for high-sensitivity cardiac troponin: is less more? Cardiovasc Ther 2019;2019:9546931.
11. García de Guadiana-Romualdo L, Morell-García D, Rodríguez-Fraga O, et al. Cardiac troponin and COVID-19 severity: results from BIOCOVID study. Eur J Clin Invest 2021;51:e13532.
12. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-280.e8.
13. Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020;314:58-62.
14. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 2020;11:29.
15. Lombardi CM, Carubelli V, Iorio A, et al. Association of troponin levels with mortality in italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol 2020;5:1274-80.
16. Pauklin S, Sernández IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. J Exp Med 2009;206:99-111.
17. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005;11:411-23.
18. Ding T, Zhang J, Wang T, et al. A multi-hospital study in Wuhan, China: protective effects of non-menopause and female hormones on SARS-CoV-2 infection. medRxiv 2020; doi: 10.1101/2020.03.26.20043943.
19. Dietl A, Zimmermann ME, Brandl C, et al. Distribution and specificity of high-sensitivity cardiac troponin T in older adults without acute cardiac conditions: cross-sectional results from the population-based AugUR study. BMJ Open 2021;11:e052004.
20. Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the multi-ethnic study of atherosclerosis (MESA). Maturitas 2018;108:37-44.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Suwalski P, Violano M, Müller M, Patriki D, Thibeault C, Quedenau C, Wang X, Karadeniz Z, Saccomanno J, Doehn JM, Hübner RH, Hinzmann B, Beer HJ, Wiggli B, Siemann S, Suttorp N, Witzenrath M, Hippenstiel S, Skurk C, Poller W, Pa-COVID Study Group, Sander LE, Kurth F, Borodina T, Guettouche T, Landmesser U, Heidecker B. Male carriers of HLA-C*04:01 have increased risk of cardiac injury in COVID-19. J Cardiovasc Aging 2022;2:33. http://dx.doi.org/10.20517/jca.2022.19
AMA Style
Suwalski P, Violano M, Müller M, Patriki D, Thibeault C, Quedenau C, Wang X, Karadeniz Z, Saccomanno J, Doehn JM, Hübner RH, Hinzmann B, Beer HJ, Wiggli B, Siemann S, Suttorp N, Witzenrath M, Hippenstiel S, Skurk C, Poller W, Pa-COVID Study Group, Sander LE, Kurth F, Borodina T, Guettouche T, Landmesser U, Heidecker B. Male carriers of HLA-C*04:01 have increased risk of cardiac injury in COVID-19. The Journal of Cardiovascular Aging. 2022; 2(3): 33. http://dx.doi.org/10.20517/jca.2022.19
Chicago/Turabian Style
Suwalski, Phillip, Michele Violano, Melina Müller, Dimitri Patriki, Charlotte Thibeault, Claudia Quedenau, Xiaomin Wang, Zehra Karadeniz, Jacopo Saccomanno, Jan-Moritz Doehn, Ralf-Harto Hübner, Bernd Hinzmann, H. Juerg Beer, Benedikt Wiggli, Sandra Siemann, Norbert Suttorp, Martin Witzenrath, Stefan Hippenstiel, Carsten Skurk, Wolfgang Poller, Pa-COVID Study Group, Leif E. Sander, Florian Kurth, Tatiana Borodina, Toumy Guettouche, Ulf Landmesser, Bettina Heidecker. 2022. "Male carriers of HLA-C*04:01 have increased risk of cardiac injury in COVID-19" The Journal of Cardiovascular Aging. 2, no.3: 33. http://dx.doi.org/10.20517/jca.2022.19
ACS Style
Suwalski, P.; Violano M.; Müller M.; Patriki D.; Thibeault C.; Quedenau C.; Wang X.; Karadeniz Z.; Saccomanno J.; Doehn J.M.; Hübner R.H.; Hinzmann B.; Beer HJ.; Wiggli B.; Siemann S.; Suttorp N.; Witzenrath M.; Hippenstiel S.; Skurk C.; Poller W.; Pa-COVID Study Group.; Sander LE.; Kurth F.; Borodina T.; Guettouche T.; Landmesser U.; Heidecker B. Male carriers of HLA-C*04:01 have increased risk of cardiac injury in COVID-19. J. Cardiovasc. Aging. 2022, 2, 33. http://dx.doi.org/10.20517/jca.2022.19
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.